Mechanical fuel pump pressure regulator
Lewis Dot Structure for Boron Oxide (B2O3) Boron (metal) has 3 valence electrons Oxygen (nonmetal) has 6 valence electrons, needs 2 to be balanced B3↘ ↙O2 B2 O3 Lewis Dot Structure for Sodium Fluoride Na +1 Cl-1 Na has 1 valence electron Fl needs 1 to be balanced Na Cl Covalent Bonds Covalent Bonds are attractions of 2 nonmetals They have low melting/boiling points and aren't a good ... BCl3. Back. 70 More Lewis Dot Structures. Note**Boron is an exception and will not form an octet. Contains 3 bonding pairs and no lone pairs. This correlates with the property that it is dangerously reactive. Note- BF3,BBr3,BI3 are the same shapes.
The Na + cation and the Cl-anion are held together by electrostatic or ionic bonds.There is no sharing in ionic bonding. The anion takes the electron for itself and the cation is happy to get rid of its electron. The ions in ionic compounds are arranged in three-dimensional structures. Lewis Electron Dot Structure Calculator. What is the lewis dot structure for ozone? Chemistry stack exchange electron (lewis) structures pages 1 4 flip pdf download fliphtml5 why hcooh written like bottom but not top? Doesn t top satisfy octet rule?: chemhelp formation of kcl with an structure? Quora diagram chemical bonds full version hd quality diagramtonyb nowroma it.
|
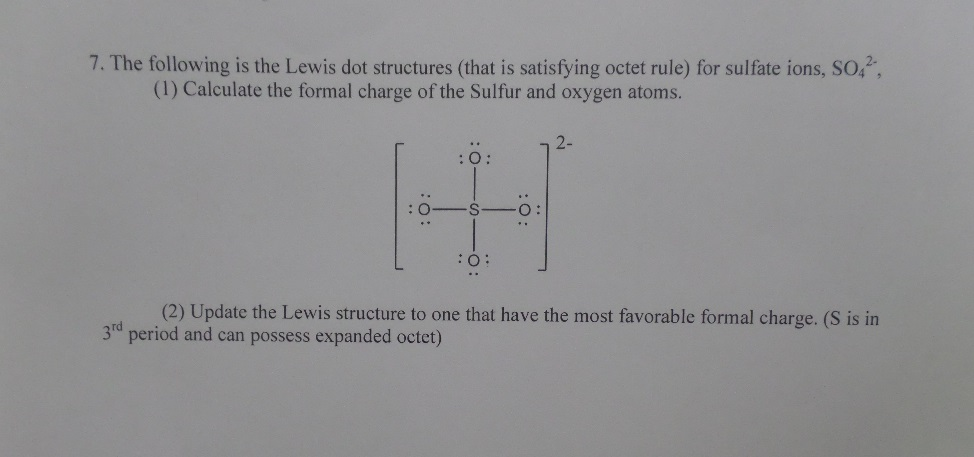
Lewis Dot Structure For Ionic Compounds Calculator Answer
As a point of reference, the bond length in N2 is 109.8 pm. (c) In ionic azides, such as NaN3, the N–N bond lengths in the azide anion are equal; both are 116 pm. Draw a Lewis structure including all resonance structures and nonzero – formal charges for the isolated azide anion, N3 and explain why the bond lengths are equal in this. Get the free 'Lewis structure' widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram Alpha.
